The Dawn of MicroRNAs – A Tiny Discovery with Massive Implications
The Nobel Prize has long honored breakthroughs that redefine human knowledge. But in 2024, the awards did something unprecedented: they celebrated science that thrives at the intersections of disciplines. This year’s laureates—Victor Ambros, Gary Ruvkun, John Hopfield, and Geoffrey Hinton—embody how curiosity and persistence in seemingly niche fields can ripple across biology, physics, and artificial intelligence (AI). Let’s unravel their stories, the science behind their work, and why their “small” discoveries changed everything.
MicroRNAs – Small Molecules, Big Revolution
The Accidental Discovery in a Tiny Worm
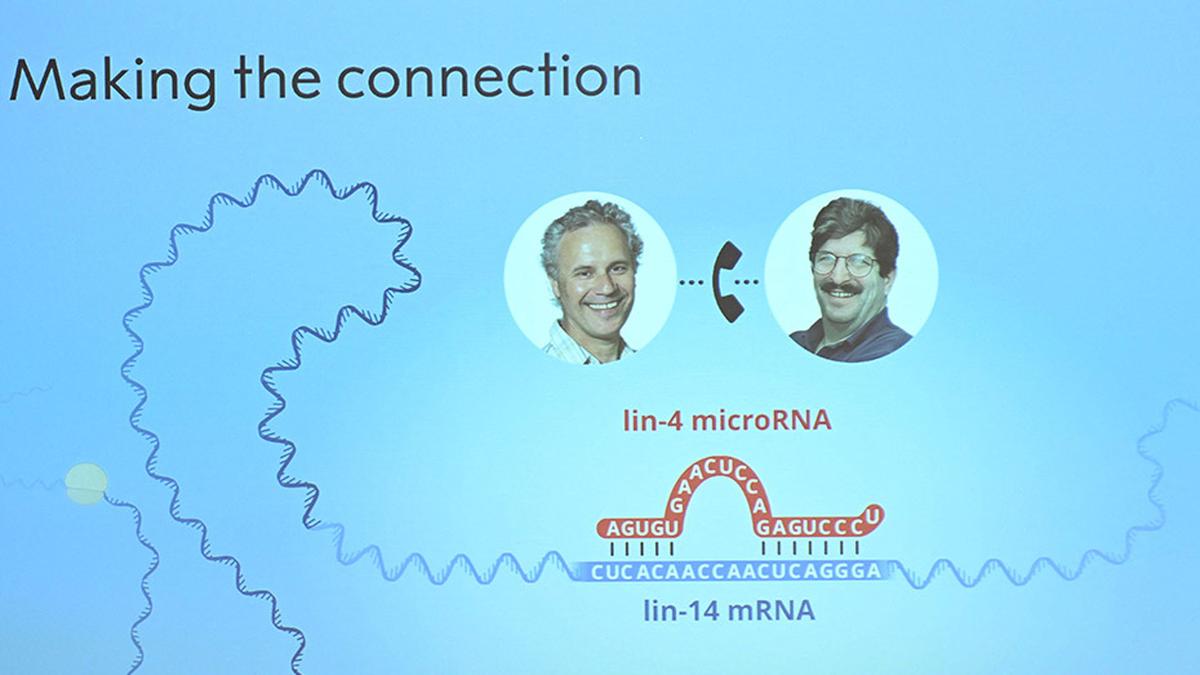
Our story begins in the early 1990s, in a Harvard lab where Victor Ambros studied C. elegans, a transparent roundworm no longer than a pencil tip. Ambros was investigating how these worms transitioned from larvae to adults when he stumbled upon a genetic oddity: a tiny RNA molecule called lin-4.
At the time, biologists believed RNA’s main role was to serve as a messenger (mRNA) carrying instructions from DNA to build proteins. But lin-4 didn’t code for a protein. Instead, it silenced another gene, lin-14, by binding to its mRNA—like a molecular “brake pedal” controlling development timing.
When Ambros and colleague Gary Ruvkun published their findings in 1993, skepticism flooded in. How could a snippet of RNA, just 22 nucleotides long, regulate genes? Critics dismissed it as a quirk of worms, irrelevant to humans.
From Skepticism to Breakthrough: The Role of let-7
The tide turned in 2000, when Ruvkun’s team discovered let-7, another microRNA. Unlike lin-4, let-7 was conserved across species—from worms to fruit flies to humans. This meant evolution had preserved its role for over 600 million years, hinting at its critical function. Suddenly, microRNAs weren’t a curiosity; they were a universal language of gene regulation.
Researchers soon found microRNAs everywhere:
Cancer: Tumors often have abnormal microRNA levels, which silence tumor-suppressor genes.
Heart Disease: MicroRNAs regulate cardiac muscle repair.
Viruses: Hepatitis C hijacks human microRNAs to replicate.
By the 2020s, microRNA-based therapies entered clinics. Drugs like Miravirsen, which blocks a liver-specific microRNA to treat hepatitis C, and melanoma therapies targeting oncogenic miRNAs, became reality. Yet, the Nobel Committee waited—they wanted to see real-world impact.
MicroRNAs in Medicine: From Theory to Therapy
Today, microRNAs are indispensable in diagnostics and treatment. For example:
1.Early Cancer Detection: Blood tests detect miRNA signatures unique to pancreatic or breast cancer.
2.Customized Therapies: miRNAs can be engineered to silence disease-causing genes, offering precision medicine.
This quiet revolution in biology began with a humble worm—and two scientists who refused to let skepticism derail them.
Neural Networks – Bridging Physics and Artificial Intelligence
Hopfield’s Brain-Inspired Physics Model
In 1982, physicist John Hopfield asked a radical question: Could the brain’s neural networks be modeled using physics? Drawing inspiration from spin glasses—metals with disordered magnetic fields—he designed the Hopfield network, a mathematical model where artificial neurons “fire” based on input from neighbors.
This network had a remarkable property: it could store memories. Like how a magnet’s state influences its neighbors, neurons in a Hopfield network stabilize into patterns, mimicking associative memory (e.g., recalling a face from a partial image). Though groundbreaking, it was seen as a theoretical toy—after all, the 1980s were the height of the AI winter, a period of disillusionment with artificial intelligence.
Hinton’s Persistence Through the AI Winter
Enter Geoffrey Hinton, a British psychologist turned computer scientist. While others abandoned neural networks, Hinton bet on their potential. In 1986, he (with David Rumelhart and Ronald Williams) popularized backpropagation, an algorithm for training multi-layered networks by propagating errors backward—like a teacher correcting a student’s mistakes.
Critics argued backpropagation was too computationally heavy. But Hinton persisted. His breakthrough came in 2012, when his student Alex Krizhevsky used graphics processing units (GPUs) to train a deep neural network, AlexNet, which crushed competitors in an image recognition contest. Suddenly, the world woke up to deep learning.
The Perfect Storm: GPUs, Data, and Deep Learning’s Rise
Three factors ignited the AI revolution:
GPUs: Their parallel processing power made training large networks feasible.
Big Data: The internet provided vast datasets for training.
Improved Algorithms: Techniques like convolutional neural networks (CNNs) optimized image analysis.
By the 2020s, neural networks could:
- Diagnose diseases from X-rays better than doctors.
- Power self-driving cars.
- Predict protein structures (see AlphaFold), accelerating drug discovery.
Hopfield’s physics-inspired model and Hinton’s relentless optimization had turned AI from a pariah to a powerhouse.
When Biology Meets AI – A Nobel-Worthy Convergence
MicroRNAs as Diagnostic Powerhouses
By the 2020s, microRNAs became cornerstones of liquid biopsies—non-invasive tests detecting cancer from a blood drop. For example, miR-21 is overexpressed in breast cancer, while miR-122 predicts liver damage. These tiny molecules offered a window into our health, enabling early interventions.
Neural Networks Solving Physics’ Biggest Puzzles
Meanwhile, AI began tackling physics problems:
Quantum Chemistry: Neural networks simulate molecular interactions, speeding up drug design.
Particle Accelerators: AI optimizes experiments at CERN, reducing energy waste.
Climate Modeling: Deep learning predicts extreme weather patterns.
Hopfield’s networks even found new life in energy-based models, improving AI’s efficiency.
The 2024 Nobel Decision: Rewarding Boundary-Crossing Science
In October 2024, the Nobel Committee broke tradition by awarding:
Physiology/Medicine to Ambros and Ruvkun for revealing “a new universe of genetic regulation.”
Physics to Hopfield and Hinton for models that “transformed our understanding of complex systems.”
The message was clear: the future of science lies in collaboration across silos.
Legacy and Controversy: What the Future Holds
The decision sparked debate. Had the Nobels stretched too far? Yet, it underscored a truth: the 21st century’s grand challenges—climate change, pandemics, quantum computing—demand interdisciplinary thinking.
MicroRNAs and neural networks exemplify this. Once niche, they now underpin CRISPR gene editing, AI-driven drug discovery, and sustainable energy solutions. As Hopfield once said, “Ideas that seem useless today may light tomorrow’s path.”






0 Comments